A metalloid is a chemical element that primarily exhibits properties lying between metals and nonmetals or combines aspects of both. The word metalloid is derived from the Latin metallum (meaning ‘metal”) and the Greek oeides (meaning ‘resembling in form or aspect’).
There is no universally accepted definition of a metalloid, and there is no complete consensus on which elements fall into this category. Despite its lack of specificity, the term continues to appear in the literature.
In this reading, we’ll explore what a metalloid is, its properties, diagram, examples, and application. We’ll also learn the difference between a metal, nonmetal, and metalloid.
Let’s get started!
Read about Graphite with this detailed guide!
What Is Metalloid?
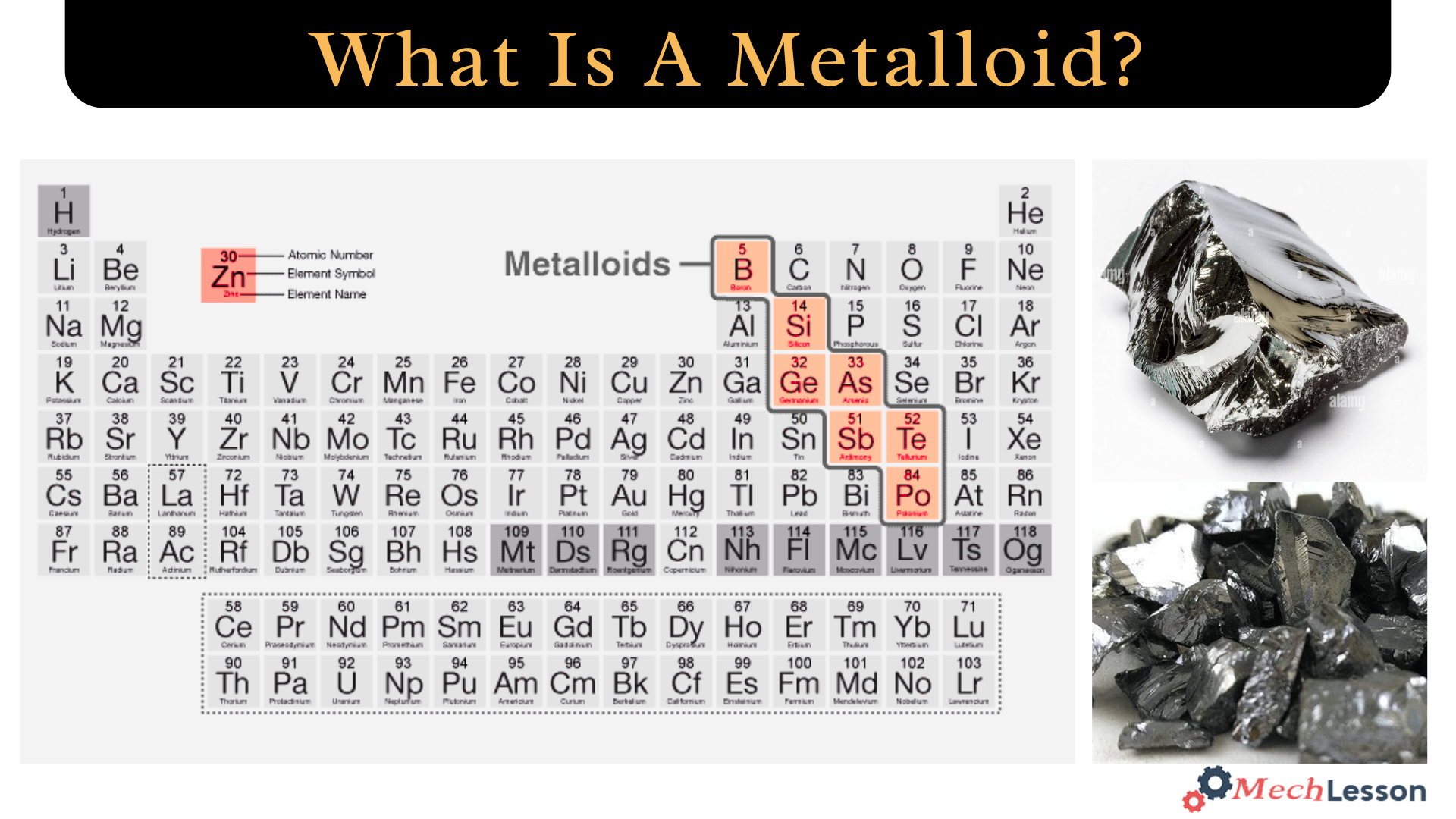
Metalloids are chemical elements that sit somewhere between the metal and non-metal classifications in terms of their chemical and physical properties. The seven most well-known metalloids are polonium, arsenic, tellurium, silicon, antimony, boron, and germanium.
The standard periodic table shows that all seven of these elements are located in a diagonal section of the p-block that runs from astatine (located on the lower right) to boron (located on the upper left). The metalloids are located below the line that separates metals from nonmetals in some periodic tables.
Metalloids are typically fragile and only passably good electrical conductors, despite their metallic look. These elements typically exhibit non-metal behavior in chemistry. It is possible for metalloids to create metallic alloys.
In most cases, the metalloid elements’ physical and chemical properties fall somewhere in the middle. These components generally have few structural uses because they are very brittle. Metalloids and related compounds find use in semiconductors, pyrotechnics, biological agents, glasses, flame retardants, alloys, catalysts, optical storage, and optoelectronics.
Properties Of Metalloid
Here are the common properties of a metalloid:
- Metalloids usually have a metal-like appearance. These elements, on the other hand, frequently exhibit non-metal behavior.
- Electronic band structures of metalloids are known to resemble those of semiconductors or semimetals.
- Metalloids have a brittle, slightly glossy appearance and are often solid at room temperature.
- These elements typically behave chemically as nonmetals (although weakly).
- These elements can form metallic alloys. Metalloids essentially have several intermediate physical and chemical properties.
- The electrical conductivity of these elements is typically intermediate to fairly strong.
- Metalloids can form amphoteric or weakly acidic oxides.
- These elements have intermediate ionization energies and electronegativity values.
Read about What are Metals, Their Properties and Classification? with this detailed guide!
Metalloid Diagram
You should also read about Metals with this detailed guide!
Examples Of Metalloid
One example of a typical metalloid is silicon. It is brittle like a nonmetal yet has the sheen of a metal. Silicon’s electrical conductivity falls between that of a metal and a nonmetal, which makes it a popular material for computer chips and other electronics.
Boron is versatile and can be used in many compounds (see image). The thermal shock resistance of borosilicate glass is exceptionally high. When borosilicates are exposed to extreme temperature variations, the material will not be harmed, compared to other glass compositions that might break or crack. Golf clubs, fishing rods, and airplanes all use boron filaments because of their strength and lightweight. Fiberglass and a variety of detergents and cleansers both use sodium tetraborate extensively as insulation.
The use of arsenic to carry out the heinous act has long been a factor in murder mysteries. Arsenic is clearly detectable during autopsy; therefore, this use of the substance is not very wise. Pesticides, herbicides, and insecticides include arsenic; however, because of the metal’s toxicity, its use in these products is declining. The use of arsenic as a wood preservative stems from its efficacy as an insecticide.
The bluish-white, brittle metal antimony is a poor electrical conductor. When used with lead, antimony increases the mixture’s hardness and strength. This material is essential for the production of semiconductors and electronic devices. Approximately 50% of the industrially used antimony is used to make alloys, batteries, and bullets.
Application Of a Metalloid
Biological agents (which can also be nutritional, toxicological, and medicinal), flame retardants, catalysts, glasses (which can be metallic or oxides), optical storage media, and alloys (or in the production of alloys as a component of the mixture) are all common applications for metalloids and their compounds.
Metalloids are also employed in electronics, pyrotechnics, optoelectronics, and semiconductors. When it comes to lighter metalloids, alloys generated by the combination of transition metals are exceptionally well represented.
It is possible for boron to create intermetallic compounds. If n is more than 2, this element can also combine with these metals with MnB composition to make alloys. Actually, boron may be injected into steel using ferroboron, which has a 15% boron content.
In the engineering industry, nickel-boron alloys are also used as components for case hardening compositions and welding alloys. The automotive and construction industry make extensive use of silicon alloys made of iron and aluminum. It is known that germanium may create a variety of alloys, particularly those related to coinage.
Difference Between a Metal, Non-metal & Metalloid
When comparing the three sets of elements on the periodic table, the metals serve as a benchmark because they constitute the biggest group. The properties of nonmetals and metalloids are determined by how they compare to metals. Here are the differences between metal, non-metal and metalloid based on their ductility, appearance, and malleability:
Read about Bronze with this detailed guide!
Ductility
- Metals: Metals are extremely ductile and will not break when pulled into wire.
- Nonmetals: These materials are not ductile and cannot be pulled into wire without shattering.
- Metalloids: While some metalloids are ductile, some are not.
Appearance
- Metals: The majority of metals are reflective and have a gleaming metallic sheen.
- Nonmetals: Nonmetals lack a metallic sheen and are typically dull or nonreflective.
- Metalloids: Depending on the element, metalloids can appear metallic or nonmetallic.
Malleability
- Metals: The majority of metals are pliable and resistant to breaking when rolled or pressed into various shapes. A metal’s atoms can easily slip past one another because of its regular, repeating pattern.
- Nonmetals: Generally speaking, nonmetals are not very bendable and will shatter when rolled or struck. In a nonmetal, the atoms are not regular in their arrangement and do not readily slide past one another.
- Metalloids: While some metalloids are malleable, some are not. One metalloid that is not particularly malleable is silicon, but boron is a metalloid that is highly malleable.
Read about The Difference Between Metal and Non-Metals with this detailed guide!
FAQs
What are the 7 metalloids?
In the present-day periodic table, you may find seven metalloid elements. Boron (B), Silicon (Si), Germanium (Ge), Arsenic (As), Antimony (Sb), Tellurium (Te), and Polonium.
What defines a metalloid?
The term “metalloid” refers to an element that is difficult to categorize because it has properties that are similar to, or even identical to, those of both metals and nonmetals.
What are five characteristics of metalloids?
- Metalloids are solids.
- Metalloids have low elasticity.
- They are brittle.
- Semiconductive.
- Average heat conductivity.
What are the differences between metals and metalloids?
- Metalloids may transfer heat and electricity, but not quite to the same extent as metals.
- Metalloids are not as hard as nonmetals, and they are more prone to breaking or shattering.
- Metalloids often have a metallic sheen and reflect light.

