Metals are known to be natural compounds of the earth’s crust generally found in the form of iron ores. They are present in rocks washed by surface water and groundwater and in atmospheric dust.
They are substances that form naturally below the surface of the earth. Also, they are lustrous or shiny and are called inorganic because they are made of substances that were never alive. Well, in this reading, we’ll explore what metals are, their properties, classifications, and the periodic elements of metals.
Let’s get started!
Learn about the 23 Different Types of Metals and Their Uses with this detailed guide!
What are Metals?
Metal is any group of substances with strong thermal and electrical conductivity, malleability, ductility, and high-light reflection. Metals make up about 75 percent of all chemical elements that are currently understood. Aluminum, iron, calcium, sodium, potassium, and magnesium are the elements that are most prevalent in the crust of the Earth.
Most metals are found in ores (materials that contain minerals), although some, like copper, gold, platinum, and silver, often turn up in the free state because they do not easily react with other elements.
Usually, metals are crystalline solids. They typically have a simple crystal structure that is characterized by strong atom packing and a high level of symmetry. Metal atoms often have fewer than half of their total number of electrons in their outermost shell. This property makes metals less likely to combine to form compounds.
However, nonmetals (such as oxygen and sulfur), which typically contain more than half the maximum amount of valence electrons, communicate with them more easily. The chemical reactivity of different metals varies greatly. Lithium, potassium, and radium are among the most reactive; gold, silver, palladium, and platinum are among the least reactive.
The free-electron theory offers the best reason for the high electrical and thermal conductivities of the simple metals (i.e., the non-transition metals of the periodic table).
According to this theory, the individual atoms in these metals have lost their valence electrons to the solid as a whole. The free electrons that result from this loss are what cause conductivity, and they move as a group inside the solid.
The band theory, which considers both the presence of free electrons and their interaction with so-called d electrons, is a better explanation for conductivities in the case of the more complex metals (i.e., the transition elements).
It is common to attribute the mechanical properties of metals, such as hardness, durability under repeated stress (fatigue strength), ductility, and malleability, to flaws or faults in their crystal structure.
For instance, a metal can deform plastically and avoid being brittle since there isn’t an atom layer in its densely packed structure.
Learn about Plastics with this detailed guide!
What are the Properties of Metals?
The properties of metals include mechanical, physical and chemical properties.
Mechanical Properties
The ability of a material to stand up to various external forces, such as shear stresses, loads, environmental factors, and time, is known as its mechanical property.
The metal’s durability against shearing, stretching, twisting, and compression as well, or breaking under a specific set of situations, can be measured by mechanical engineers.
Once identified, these mechanical properties can be used to judge a material’s fitness for a certain activity as well as its capacity to handle abrupt loads and pressures. The most frequent tests used to ascertain a metal’s features are listed below.
Physical Properties
With the exception of mercury, which is liquid at room temperature (Gallium is liquid on hot days), all metals are solid at room temperature.
Metals are good conductors of heat and electricity, which is why most cooking utensils are made up of iron and iron is made up of metals. Also, metals are ductile in nature and this is their ability for them to be stretched into wire.
With the durability of drawn wires, they are used to make cranes, cable wires and soldering applications. With this, metals are said to be ductile. Furthermore, metals are malleable, which allows them to be beaten into flat sheets. Most common of them are aluminum sheets.
Aluminum sheets are used in the manufacturing of aircraft because of their lightweight and strength. They are also used in automobile industries for making car body parts. Well, this is because of the malleability of metals.
Metals are said to be sonorous due to their production of a deep ringing sound when struck with other solid objects. Also, metal’s physical properties can be seen in their shiny appearance, although they can be polished to have a shiny appearance.
Learn about Non-Ferrous Metals with this detailed guide!
Chemical Properties
One of the common chemical properties of metals is that they react with water. Although not all kinds of metals do react, the highly reactive ones like sodium can react with water and oxygen. During the process, sodium will release a large amount of heat.
Metals can also react with acid to produce hydrogen gas. Zinc reacts with hydrochloric acid to produce zinc chloride and hydrogen gas. Also, metals can react with bases to produce metal salts and hydrogen gas.
For instance, when zinc reacts with strong hydroxide, it offers sodium zincate and hydrogen gas.
Finally, metals can react with oxygen to produce metal oxide, that is, when burned together. These metal oxides are basic in nature. For instance, a magnesium strip burned in the presence of oxygen forms magnesium oxide. Also, when magnesium oxide dissolves in water, it forms magnesium hydroxide.
Classifications of Metals
Metals can be classified according to the following:
- Iron content
- Atomic structure
- Magnetic and non-magnetic metals
Classification by Iron Content
Iron content is the common way of classifying metals. Metals that contain iron are known as ferrous metals. The iron type imparts magnetic properties to the material and also makes them prone to corrosion when exposed to moisture.
On the other hand, metals that do not have any iron content in them are known as non-ferrous metals. They do not have any magnetic properties. Examples of non-ferrous metals include aluminum, lead, brass, copper, and zinc.
Learn about How Metals React to Weather Conditions & How to Resolve Them with this detailed guide!
Classification of Metals According to Their Atomic Structure
Metals may also be classified based on their atomic structure stated in the periodic table. They may be known as alkaline, alkaline earth, or transition metals belonging to the same group that behaves similarly when reacting with other elements. That is to say, they have similar chemical properties.
The alkali metals are in group IA on the far left side of the periodic table. They are known to be highly reactive elements and distinctive because of their +1 oxidation state and they have low density compared to other metals. Because of their reactivity, they are elements found in most compounds.
The alkaline earth metals are found in group IIA of the periodic table, located in the second column of the table. They have a +2 oxidation state and like the alkali metals, they are found in compounds rather than in pure form.
Alkaline earth metals are also reactive but not as reactive as alkali metals. The group IIA metals are hard and shiny and usually malleable and ductile.
Lastly, basic metals are used to associate the term metal as they conduct heat and electricity. They have a metallic luster and tend to be dense, malleable, and ductile. Although some of these metals behave as non-metallic, allotropes of tin, for instance, act more as nonmetals.
Lead and gallium are examples of elements that are soft because they have lower melting and boiling points than other metal types.
Classification According to Magnetic and Non-Magnetic Metals
Another best way of differentiating metals is by checking how they react to magnets. In fact, it’s highly possible to differentiate metal types as magnetic and non-magnetic, since ferromagnetic metals attract well and strongly to magnets. On the other hand, paramagnetic ones show weak interactions with magnets. Lastly, there is a group of metals that shows weak repulsion to magnets.
Now let’s dive into popular types of metals and their properties.
Metals available out there share some similar mechanical properties, but when closely looked at, one must have a slightly different one from the other. there are possible ways of tweaking the properties of metals to get the desired property.
There are things to be considered when selecting metal for a particular application. These factors may include:
- Melting point
- Cost, ease of machining
- Space available
- Sufficient safety factor
- Temperature coefficient
- Density, and
- Thermal and electrical conductivity.
Learn about the Difference Between Ferrous and Non-Ferrous Metals with this detailed guide!
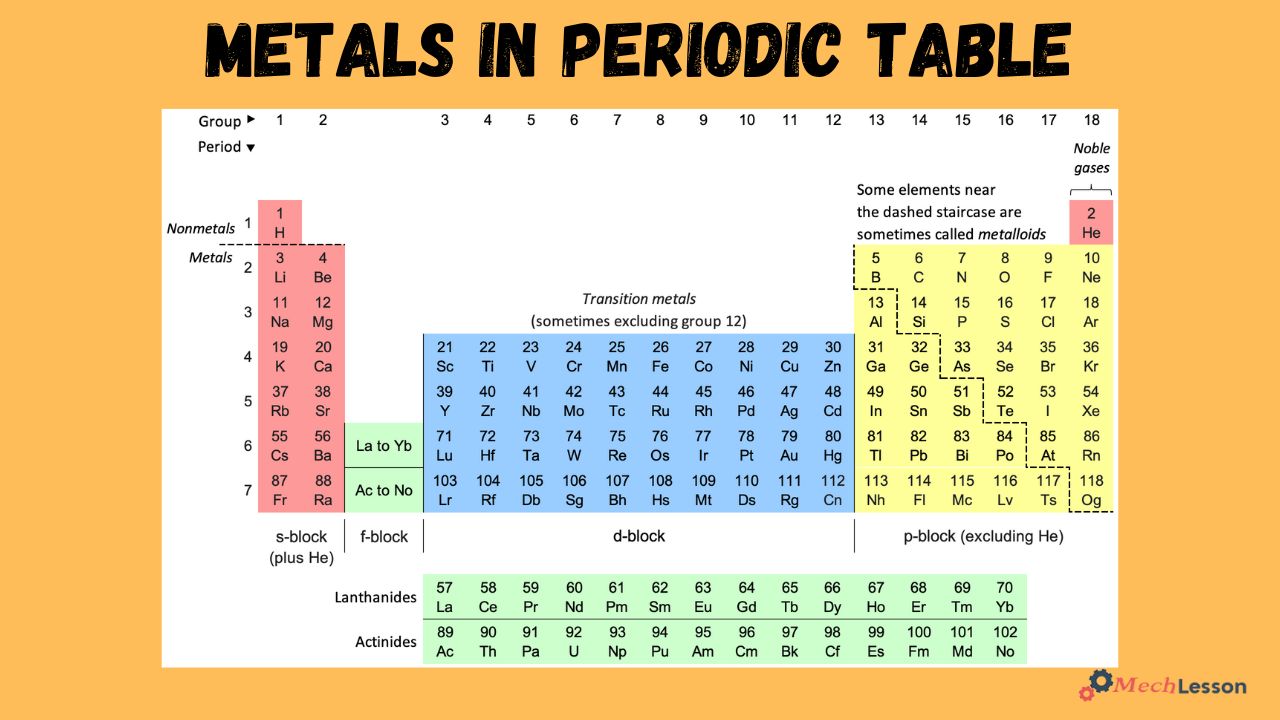
Metals in the Periodic Table
Most elements on the periodic table are metals, as they are grouped according to their types. Metals are labelled blue, non-metals yellow, or metalloids red.
The metal elements are grouped together on the left-hand side of the periodic table. Hydrogen, which is the only nonmetal grouped among the metals in the top left corner. This is because some scientists discover that there could be metallic hydrogen in the core of the planet jupiter.
Metals are remarkably useful based on how the metal atoms bond with each other, known as metallic bonding. This metallic bonding is how metal atoms interact on the atomic level and how they connect to make larger metal structures.
Metal atoms share their outermost electrons evenly with each other, which is known as a sea of electrons. The electrons flowing between the positively charged ions transmit heat and electricity with ease. This makes metals good conductors of heat and electricity.
The reactivity of metals is the tendency of metals to react with chemicals in their surroundings. Metal reactivity can vary greatly, as those in columns 1 and 2 on the periodic table react easily with many other chemicals. Although they are rarely found in their pure, elemental form.
Learn about Tensile Test with this detail guide!
Different Types of Metals in the Periodic Table
The table below shows the different elements of metal in the periodic table, explaining their atomic number, symbol, and metal elements.
| S.No | Atomic Number | Symbol | Metal Elements |
| 1 | 3 | Li | Lithium |
| 2 | 4 | Be | Beryllium |
| 3 | 11 | Na | Sodium |
| 4 | 12 | Mg | Magnesium |
| 5 | 13 | Al | Aluminium |
| 6 | 19 | K | Potassium |
| 7 | 20 | Ca | Calcium |
| 8 | 21 | Sc | Scandium |
| 9 | 22 | Ti | Titanium |
| 10 | 23 | V | Vanadium |
| 11 | 24 | Cr | Chromium |
| 12 | 25 | Mn | Manganese |
| 13 | 26 | Fe | Iron |
| 14 | 27 | Co | Cobalt |
| 15 | 28 | Ni | Nickel |
| 16 | 29 | Cu | Copper |
| 17 | 30 | Zn | Zinc |
| 18 | 31 | Ga | Gallium |
| 19 | 37 | Rb | Rubidium |
| 20 | 38 | Sr | Strontium |
| 21 | 39 | Y | Yttrium |
| 22 | 40 | Zr | Zirconium |
| 23 | 41 | Nb | Niobium |
| 24 | 42 | Mo | Molybdenum |
| 25 | 43 | Tc | Technetium |
| 26 | 44 | Ru | Ruthenium |
| 27 | 45 | Rh | Rhodium |
| 28 | 46 | Pd | Palladium |
| 29 | 47 | Ag | Silver |
| 30 | 48 | Cd | Cadmium |
| 31 | 49 | In | Indium |
| 32 | 50 | Sn | Tin |
| 33 | 55 | Cs | Cesium |
| 34 | 56 | Ba | Barium |
| 35 | 57 | La | Lanthanum |
| 36 | 58 | Ce | Cerium |
| 37 | 59 | Pr | Praseodymium |
| 38 | 60 | Nd | Neodymium |
| 39 | 61 | Pm | Promethium |
| 40 | 62 | Sm | Samarium |
| 41 | 63 | Eu | Europium |
| 42 | 64 | Gd | Gadolinium |
| 43 | 65 | Tb | Terbium |
| 44 | 66 | Dy | Dysprosium |
| 45 | 67 | Ho | Holmium |
| 46 | 68 | Er | Erbium |
| 47 | 69 | Tm | Thulium |
| 48 | 70 | Yb | Ytterbium |
| 49 | 71 | Lu | Lutetium |
| 50 | 72 | Hf | Hafnium |
| 51 | 73 | Ta | Tantalum |
| 52 | 74 | W | Tungsten |
| 53 | 75 | Re | Rhenium |
| 54 | 76 | Os | Osmium |
| 55 | 77 | Ir | Iridium |
| 56 | 78 | Pt | Platinum |
| 57 | 79 | Au | Gold |
| 58 | 80 | Hg | Mercury |
| 59 | 81 | Tl | Thallium |
| 60 | 82 | Pb | Lead |
| 61 | 83 | Bi | Bismuth |
| 62 | 84 | Po | Polonium |
| 63 | 87 | Fr | Francium |
| 64 | 88 | Ra | Radium |
| 65 | 89 | Ac | Actinium |
| 66 | 90 | Th | Thorium |
| 67 | 91 | Pa | Protactinium |
| 68 | 92 | U | Uranium |
| 69 | 93 | Np | Neptunium |
| 70 | 94 | Pu | Plutonium |
| 71 | 95 | Am | Americium |
| 72 | 96 | Cm | Curium |
| 73 | 97 | Bk | Berkelium |
| 74 | 98 | Cf | Californium |
| 75 | 99 | Es | Einsteinium |
| 76 | 100 | Fm | Fermium |
| 77 | 101 | Md | Mendelevium |
| 78 | 102 | No | Nobelium |
| 79 | 103 | Lr | Lawrencium |
| 80 | 104 | Rf | Rutherfordium |
| 81 | 105 | Db | Dubnium |
| 82 | 106 | Sg | Seaborgium |
| 83 | 107 | Bh | Bohrium |
| 84 | 108 | Hs | Hassium |
| 85 | 109 | Mt | Meitnerium |
| 86 | 110 | Ds | Darmstadtium |
| 87 | 111 | Rg | Roentgenium |
| 88 | 112 | Cn | Copernicium |
| 89 | 113 | Nh | Nihonium |
| 90 | 114 | Fl | Flerovium |
| 91 | 115 | Mc | Moscovium |
| 92 | 116 | Lv | Livermorium |
Conclusion
Metals are a fundamental group of materials known for their high strength, durability, conductivity, ductility, and lustrous appearance. They are crucial in virtually every industry—ranging from construction and manufacturing to electronics and transportation.
Metals can be classified into pure metals and alloys, and also into ferrous (iron-containing) and non-ferrous types. Their physical and chemical properties make them indispensable for producing tools, machines, structures, and countless everyday objects. Understanding metals is essential for selecting the right material for any engineering or industrial application.
You should also learn about Toughness, Hardness and Strength in a Material with this detailed guide!
FAQs on Metals
What are metals?
Metals are solid elements known for their high thermal and electrical conductivity, malleability, ductility, and metallic luster.
What are the main types of metals?
Metals are mainly classified as ferrous (contain iron, like steel) and non-ferrous (do not contain iron, like copper and aluminum).
What are alloys?
Alloys are mixtures of metals or a metal with another element, designed to improve specific properties. Examples include steel, brass, and bronze.
Which metals are the best conductors of electricity?
Silver is the best, followed by copper and gold.
Why are metals used in construction?
Due to their high strength, durability, and load-bearing capacity, metals like steel are widely used in buildings, bridges, and infrastructure.
Are all metals magnetic?
No. Only some metals like iron, nickel, and cobalt, are naturally magnetic.
What makes stainless steel rust-resistant?
The addition of chromium in stainless steel forms a protective oxide layer, preventing rust and corrosion.

