In electrochemical machining, electrically conductive material is removed from a workpiece by grinding it with a negatively charged abrasive wheel, which ensures that the materials removed remain in the electrolyte fluid.
ECG is a hybrid process that combines electrochemical machining and grinding, with the cutting tool serving as the cathode and the workpiece as the anode. In this reading, we’ll examine electrochemical grinding’s definition, uses, components, diagram, and operation, as well as the benefits and drawbacks of ECG.
Let’s begin!
Learn about grinding machines with this detailed guide!
What is Electrochemical Grinding?
In electrochemical grinding (ECG), a grinding wheel is used in the precision machining technique to remove material from a workpiece’s surface. This method achieves exceptional precision and efficiency by using conventional grinding techniques with electrochemical machining for high-hardness workpieces, where traditional techniques can be difficult and the ECG works very well.
An electrolyte fluid is used to wash away the material after it has been taken from the anode. This occurs after mechanically cutting the metal with the grinding wheel and electrochemically dissolving the substance at the same time.
The ECG can lower the force of cutting because the grinding wheel can partially break down the material and lessen its hardness when grinding. The process requires fixtures made of corrosion-resistant material and electrical contact with the workpiece, although it follows some of the same guidelines as traditional grinding, including programming and setup.
The workpiece is precisely shaped and finished after the metal surface undergoes electrochemical processes that turn it into oxide, which is then removed by the revolving grinding wheel.
This machining process can achieve superior surface finishes and tight tolerances, which is why it is known as the reverse electroplating method. It provides a sophisticated solution for difficult machining requirements.
The Electrolyte
Electrolyte has a direct impact on the anode electrochemical reaction; this is why its selection is essential for the electrochemical grinding process. It should stop machine rust and be safe for human health.
A rich source, low cost, good economic impact, ease of consumption during processing, non-toxicity to the human body, no dusting of equipment and fixtures, good conductivity for high productivity, and good surface roughness and dimensional accuracy are some of the criteria for choosing an electrolyte.
Applications
Below are the applications of electrochemical grinding (ECG):
- ECG is used in aerospace, power generation, and manufacturing for accurate grinding of hard surfaces, honeycomb structures, and complex turbine blades.
- The process is ideal for grinding delicate items, producing sharp products, and finishing hard surfaces.
- It is ten times faster material removal rate than traditional machining, suitable for hard materials like exotic metals and stainless steel in electronics and medical device manufacture.
- The ECG’s low abrasion characteristics make it suitable for operations requiring minimal burrs, scratches, and residual tensions.
- It is used to eliminate surface flaws from components, like re-profiling locomotive gears.
- The process is used to eliminate fatigue fractures from undersea steel constructions using saltwater as the electrolyte and diamond particles in the grinding wheel.
Learn about Laser Beam Machining with this detailed guide!
Parts of ECG
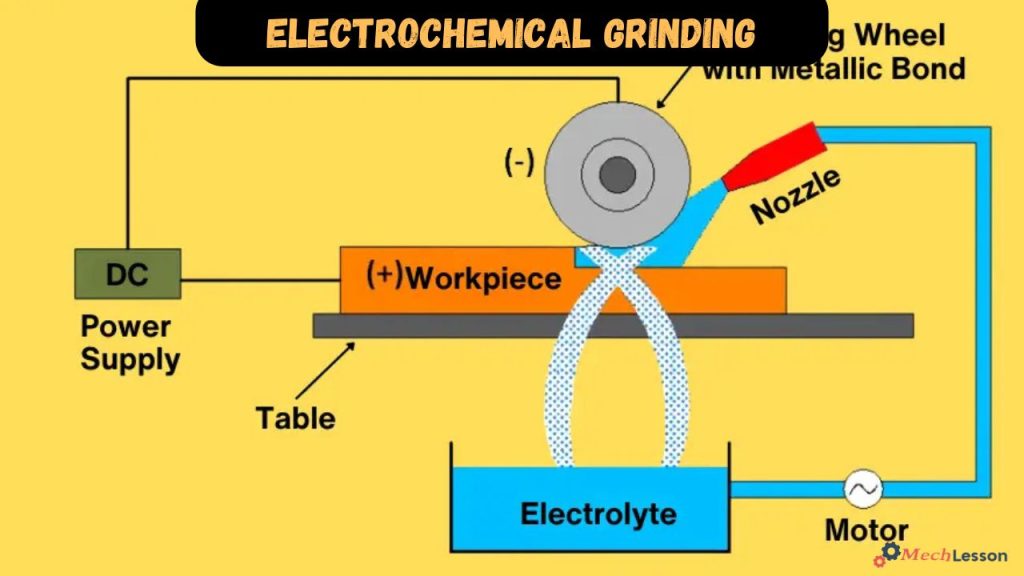
The various parts of electrochemical grinding include a DC power supply, work table and fixture, electrolyte tank, pump, filter, pressure gauge and flow meter, nozzle, sleeve, grinding wheel and collecting tank.
DC power supply: The machine setup uses a DC power supply with low voltage and high current, preventing excessive heat generation and ensuring safety during operation, while high current ensures faster and more efficient machining.
Worktable and fixture: This is a sturdy base and fixed workpiece positioning, essential for effective machining. The worktable provides rigidity and support, and fixtures firmly clamp the workpiece in place.
Electrolyte tank: An electrolyte tank is a reservoir that stores conducting solutions used during the electrochemical grinding. It completes the circuit by acting as a conducting medium and oxidizes the metal surface, carrying away oxidized particles. Common electrolytes used are Sodium compounds with electrovalent bonds, such as sodium nitrate, sodium carbonate, sodium hydroxide, and sodium chloride.
Pump: The function of an electrically driven pump is to transport electrolyte from the tank to the nozzle, ensuring a continuous flow throughout the process.
Filter: The electrolyte must undergo a filtering process to remove micro impurities, ensuring a pure electrolyte before reaching the machining area.
Pressure Gauge and flow meter: Safety equipment like pressure gauges and flow meters are used to monitor electrolyte pressure and flow. They serve as indicators so the operators can quickly turn off the equipment if these values exceed safe limits.
Nozzle: The nozzle, with a decreasing cross-section area, is crucial for accurately directing electrolyte, increasing velocity and removing material from the workpiece. Proper nozzle placement ensures the electrolyte contacts both the workpiece and the grinding wheel.
Sleeve: A sleeve is a tool that efficiently transfers electrical energy to the grinding wheel, facilitating the machining process.
Grinding wheel: The grinding wheel, a central component of an electrochemical grinding machine, serves as the cathode, connecting to the negative terminal of the power supply. It is made of insulating materials like diamond and aluminum oxide, contributing only 5-10% of material removal. The majority is achieved through electrolyte action, resulting in minimal wear.
Collecting tank: The electrolyte is collected in a designated tank after use, either for disposal or potential reuse, based on environmental and requirements considerations.
You should also learn about Ultrasonic Machining with this detailed guide!
Electrochemical Grinding Equipment
A lathe or traditional grinding machine can be used to modify electrochemical grinding equipment, or it can be professional-grade. Centrifugal pumps, tubes, nozzles, forced air extraction or neutralization equipment, filtration devices, and an adjustable voltage DC power source are all necessary. Hard metals may be shaped using this kind of grinding, which is used for tolerance.
It is a chemical reduction procedure that produces a smoother, burr-free surface and extended wheel life. Metal shapes can be customized to fit various wheel types. Compared to conventional grinding techniques, electrochemical grinding results in reduced stress on the surface.
Diagram

Learn about Numerical Control NC Machining with this detailed guide!
How Does Electrochemical Grinding Work?
For the purpose of removing metal, electrochemical grinding combines electrochemical and grinding techniques. The workpiece serves as an anode, and the grinding wheel as a cathode. The procedure makes use of electrolytes such as sodium carbonate, sodium hydroxide, sodium chlorate, and sodium nitrate.
The grinding wheel is a circular metal plate that contains abrasive particles such as silicon carbide, boron carbide, diamond dust, and aluminum oxide. The majority of the metal is removed by a reaction that happens when electrolytic fluid is injected between the workpiece and the grinding wheel. Less than 5% of the workpiece’s unwanted material is removed by the grinding wheel.
When a metal surface is exposed to an electrolyte at a high current, oxidation and the development of a corrosive oxide layer occur. This process is known as electrochemical grinding. The combined action of a revolving grinding wheel and a flowing electrolyte then removes this oxide layer.
The workpiece is fastened and firmly positioned on the worktable at the start of the procedure, leaving a thin 0.02mm space between it and the grinding wheel. The electrolyte is delivered to its assigned position and the power supply is turned on. The electrolyte is filtered to remove contaminants and its pressure is measured before it reaches the cutting area.
After gauging the electrolyte flow with a flow meter, the electrolyte is sprayed onto the workpiece. An oxide layer is created during the oxidation process and is then removed by the electrolyte flow and the grinding wheel’s abrasive particles.
You should also learn about surface roughness with this detailed guide!
Advantages
Below are the benefits of electrochemical grinding:
- Offers exceptional accuracy due to the absence of direct contact between the tool and the workpiece.
- Allows high tolerance levels for intricate, finely detailed workpieces.
- Ensures scratch-free surfaces for a smooth, flawless finish.
- Generates minimal heat energy, preventing thermal damage to the workpiece.
- Uses electrolyte as a coolant, efficiently dissipating heat.
- Extends the range of materials that can be processed efficiently.
- Delivers sharp, clean edges for high-quality workpiece features.
- Minimizes tool wear, making it suitable for machining hard materials.
Disadvantages
Below are the limitations of electrochemical grinding:
- Slow metal removal rate.
- High power consumption.
- High initial equipment cost.
- Low production rate.
- Waste electrolyte disposal.
- Large setup area.
- Requires conductive workpiece and wheel.
- Applicable only to surface grinding.
- Can cause corrosion.
- More complicated than traditional machining methods.
- Requires experienced personnel.
- Higher production costs.
Conclusion
Electrochemical Grinding (ECG) is a hybrid machining process combining electrochemical action and mechanical grinding. It is primarily used for machining hard and conductive materials that are difficult to cut using traditional methods.
ECG offers several advantages—such as minimal heat generation, no thermal damage, and no burr formation—making it ideal for applications in the aerospace, medical, and tool industries. Though it requires specialized equipment and setup, ECG provides a highly efficient and precise solution for complex parts and delicate components.
FAQs on Electrochemical Grinding (ECG)
What is Electrochemical Grinding?
ECG is a machining process that combines electrochemical action with mechanical grinding to remove material from a workpiece.
What materials can be machined using ECG?
ECG is effective on hard, conductive materials such as stainless steel, titanium, Inconel, and carbide.
How is ECG different from traditional grinding?
Unlike traditional grinding, ECG generates little to no heat, avoids mechanical stress, and produces burr-free finishes.
What are the main advantages of ECG?
Advantages include no thermal damage, excellent surface finish, longer tool life, and the ability to machine complex or delicate parts.
What industries use Electrochemical Grinding?
ECG is commonly used in the aerospace, medical, defense, and precision tooling industries.
Are there any limitations of ECG?
ECG requires conductive materials, has higher setup costs, and is limited to certain shapes and sizes.
Is ECG environmentally friendly?
ECG uses electrolytes that require careful handling and disposal, but the process itself reduces airborne particles and noise compared to traditional grinding.