Polymers are common around us today, even though there are a wide variety of different types and different classifications. In a previous post, we explained these various types of plastics as commodity plastics and specialized types.
Polymers is the common term to describe plastics; they are available in various forms and can be processed to obtain different properties. Well, in this reading, we’ll explore what a polymer is, its properties, state, and additives.
Let’s get started!
Learn about plastics with this detailed guide!
What are polymers?
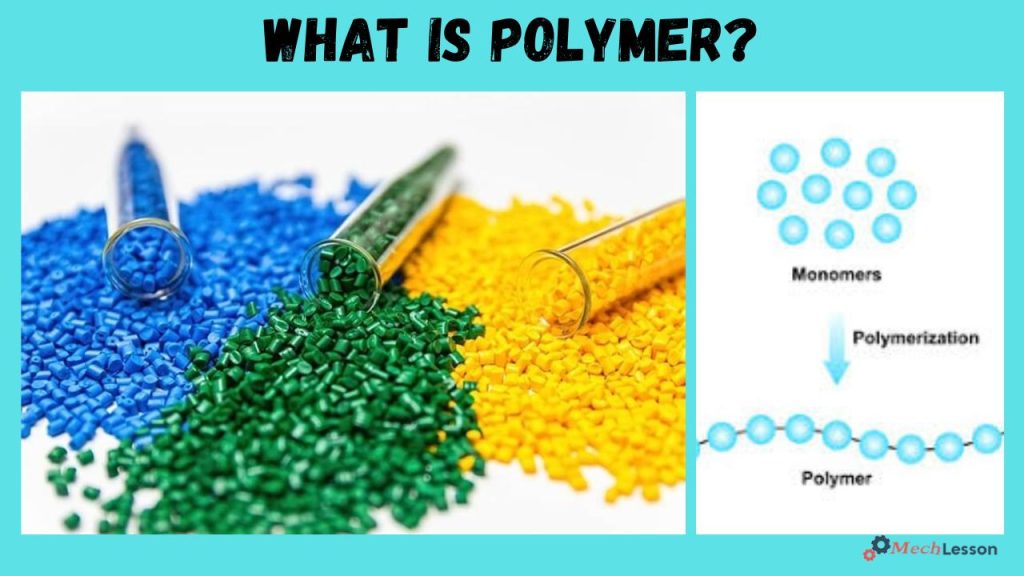
Polymers are chemical compounds whose molecules are extremely large, looking like a long chain made up of a seemingly endless series of interconnected links.
These molecules’ size is explained to be extraordinary, ranging in thousands and even millions of atomic mass units. The size of the molecule, physical state, and structure are the unique properties a plastic is known for, giving it the ability to be molded and shaped.
Thermoplastic and Thermosetting
Just as mentioned earlier, polymers that are classified as plastics are divided into two major categories: thermoplastics and thermosets.
Thermoplastics like polyethylene and polystyrene are plastics that are capable of being molded and remolded repeatedly. This is to further say a foamed-polystyrene cup can be heated and reshaped into a new form – perhaps a dish or plate.
A thermoplastic polymer structure is that of individual molecules that are separate from one another and flow past one another. These molecules may have an extremely high or low molecular weight. They may be branched or linear in structure, but still, have the feature of separability and consequent mobility. Polymers of these types are known as commodity plastics.
On the other hand, Thermosets are polymers that cannot be reprocessed through reheating. This is because, during the initial processing, thermosetting resins undergo a chemical reaction that makes them infusible and insoluble. When a polymer is produced in such a method reworking or heating may cause the application to break down.
You should learn about Thermoplastic & Thermosetting Plastic with this detailed guide!
Physical State and Molecular Morphologies of Polymers
The plastic behavior of polymers can also be influenced by their morphology, or arrangement of molecules on a large scale. So, polymer morphologies can either be amorphous or crystalline. The amorphous molecules are arranged randomly and are intertwined. Whereas crystalline molecules are arranged closely and in indiscernible order.
Thermosets are known for being amorphous, while thermoplastics are either amorphous or semicrystalline. The semicrystalline materials display crystalline regions known as crystallites within an amorphous matrix.
Thermoplastic materials are known to retain their molded shapes to a certain temperature. This is set by the glass transition temperature or the melting temperature of the particular polymer. Below the temperature is known as the glass transition temperature (Tg).
The molecules of a polymer material are frozen, which is also called the glassy state, where there is little or no movement of molecules passing one another. This makes the material more stiff and even brittle.
Above the glass transition temperature Tg, the amorphous parts of the polymer enter the rubbery state. That is, the molecules display increased mobility and the material becomes plastic and even elastic; that is, it has the ability to be stretched.
In the case of non-crystalline polymers like polystyrene, raising the temperature will further lead directly to the liquid state. On the other hand, for partly crystalline polymers such as low-density polyethylene or polyethylene terephthalate, the liquid state will not be reached until the melting temperature (Tm) is passed.
Beyond that point, the crystalline regions are no longer stable, and the rubbery or liquid polymers can be molded or extruded. Because thermosets do not melt upon preheating, they can be dimensionally stable up to a temperature at which chemical degradation begins.
Learn about Plastic Fabrication with this detailed guide!
Properties of Polymers
Below are the properties of polymers:
Physical Properties
The physical state and morphology of a polymer play a perfect role in its mechanical properties. The differences in their mechanical behavior are the elongation that takes place when plastic is loaded (stressed) in tension.
For instance, a glassy polymer such as polystyrene is quite stiff, showing a high ratio of initial stress to initial elongation. Whereas polyethylene and polypropylene, which are two highly crystalline plastics, can be used as films and molded objects. This is because their amorphous regions are well above their glass transition temperatures at room temperature.
The leathery toughness of these polymers above glass transition Tg results from the crystalline regions that exist in an amorphous, rubbery matrix. These plastics have the possibility of 100 to 1000 percent elongation.
Because the glass transition Tg of PET (another semicrystalline plastic) is above room temperature, the crystalline portions exist in a glassy matrix. Because of this, the material receives stiffness and high dimensional stability under stress, which is of great benefit in beverage bottles and recording tape.
Almost all plastics are known to exhibit certain elongation on being stressed that is not recovered when the stress is eliminated. This condition is known as “creep,” which may be very small for plastic that is well below its Tg. it can be significant for a partly crystalline plastic that is above Tg.
Mechanical Properties
The most commonly specified mechanical properties of polymers include breaking stress, stiffness, and tensile strength and are quantified in the table of properties and applications as the flexural modulus.
Toughness is another important property of a polymer, which is the energy the polymer absorbed before failure. This is often a result of sudden impact. Implementing stress repeatedly below the tensile strength of plastic may result in fatigue failure.
Almost all plastics are poor conductors of heat; conductivity can be further reduced when a gas (usually air) is induced into the material. For instance, foamed polystyrene used in cups for hot beverages has a thermal conductivity of about one-quarter of the unframed polymer.
Plastics are also electrical insulators only if designed for conductivity; besides, conductivity is important in plastics as dielectric strength (resistance to breakdown at high voltages). It’s also important as a dielectric loss (a measure of the energy dissipated as heat when an alternating current is inserted).
Learn about Metals with this detailed guide!
Polymer Additives
An additive can also be known as an ingredient combined with a polymer to arrive at a set of properties appropriate to the product. However, in many plastic products, the polymer is only one constituent. The combination of other additives is mixed during the processing and fabrication.
The following explained below are the additives used in polymer to obtain an appropriate product.
Plasticizers
The plasticizer is used to change the glass transition Tg of a polymer. For instance, polyvinyl chloride (PVC) is often mixed with nonvolatile liquids in order to change the glass transition. The vinyl siding used in homes requires an unplasticized, rigid PVC with a Tg of 85 to 90°C (185 to 195 0F). however, a PVC garden hose should remain flexible even at 0°C (32°F0).
There are many more polymers that can be plasticized, but PVC is unique in accepting and retaining plasticizers of widely varying molecular sizes and chemical compositions. The plasticizer may also take effect on the polymer’s flammability, odor, biodegradability, and even the cost of the finished product.
Colorants
Since the final appearance of plastic products must be appealing, the inclusion of colorant during the processing and fabrication is necessary. The popular pigments for coloring plastics are titanium dioxide and zinc oxide (white), carbon (black), and various other inorganic oxides like iron and chromium. Some other organic compounds can be used to add color either as pigments (insoluble) or as dyes (soluble).
Reinforcements
Just as the name suggests, reinforcements are used to improve the mechanical properties of plastics. A variety of materials, such as silica, carbon black, talc, mica, and calcium carbonate, as well as short fibers, can be incorporated as particulate fillers.
Using long or even continuous fibers as reinforcement, especially with thermosets, can be described below in fiber reinforcement.
Introducing large amounts of particulate filler during the fabrication of plastics such as polypropylene and polyethylene can increase their stiffness. The effect can be less dramatic when the temperature is below the polymer’s Tg.
Stabilizers
Stabilizers help to improve the longevity and useful life of any application. The properties of the plastic are as little as possible with time. stabilizers are added, usually in small quantities in order to counter the effects of aging. Since all carbon-based polymers are subject to oxidation, the common stabilizers used are antioxidants. Hindered phenols and tertiary amines are used in plastics in concentrations as low as a few parts per million.
You should learn The Difference Between Fabrication And Manufacturing with this detailed guide!
Conclusion
Polymers are large molecules made up of repeating structural units called monomers, bonded together through chemical processes. They can be natural (like rubber and cellulose) or synthetic (like nylon, polyethylene, and PVC), and are used across industries ranging from packaging and textiles to aerospace and biomedical fields.
Their lightweight, corrosion resistance, flexibility, and low cost make them essential materials in modern manufacturing and daily life. Understanding their types, properties, and applications is key to selecting the right polymer for specific engineering or consumer needs.
FAQs on Polymers
What is a polymer?
A polymer is a large molecule composed of many repeated subunits (monomers) bonded together to form chains or networks.
What are the main types of polymers?
The main types include thermoplastics, thermosetting plastics, elastomers, and fibers.
What is the difference between thermoplastics and thermosets?
Thermoplastics can be melted and reshaped multiple times, while thermosets harden permanently after one-time heating and molding.
Are polymers recyclable?
Thermoplastics are generally recyclable, but thermosets and some composite polymers are not easily recyclable.
What are common examples of polymers?
Polyethylene (PE), Polypropylene (PP), Polyvinyl chloride (PVC), Teflon (PTFE), and Polystyrene (PS).
What are polymers used for?
They are used in packaging, clothing, automotive parts, medical devices, electronics, and construction materials.
Are all polymers synthetic?
No. There are many natural polymers, such as proteins, DNA, cellulose, and natural rubber.